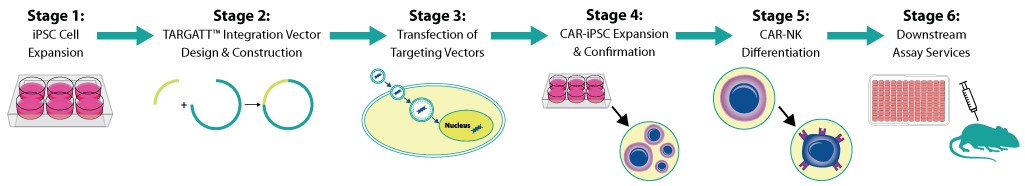
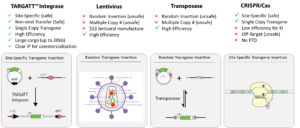
Quality GMP-Grade iPSC and Knockin-Ready TARGATT™ iPSC
To jump start iPSC derived therapeutic cell product development; achieve reliable and efficient genome integration with our innovative solutions
GMP iPSC
Our high-quality GMP-grade iPSCs (ASE-9280) were reprogrammed from CD34+ male cord blood using an episomal reprogramming method and have proven gene editing and differentiation capabilities. The ready-to-use ASE-9280 GMP line and matching research-grade iPSCs (ASE-9250) are available for purchase. Expedite your project timelines by placing your order today!
- Source cell type: CD34+ Cord Blood, healthy male
- Reprogramming method: Episomal
- Master cell bank or working cell bank available
- Seed cell bank has Drug master file with US FDA
- Matching Research iPSC Line (ASE-9250) is Available
- Proven to be genomic stable (karyotype normal) and transgene expression stable post gene editing and differentiation
- No additional passthrough cost
- Partner with CIRM (California Institute of Regenerative Medicine) in supplying GMP grade iPSC lines
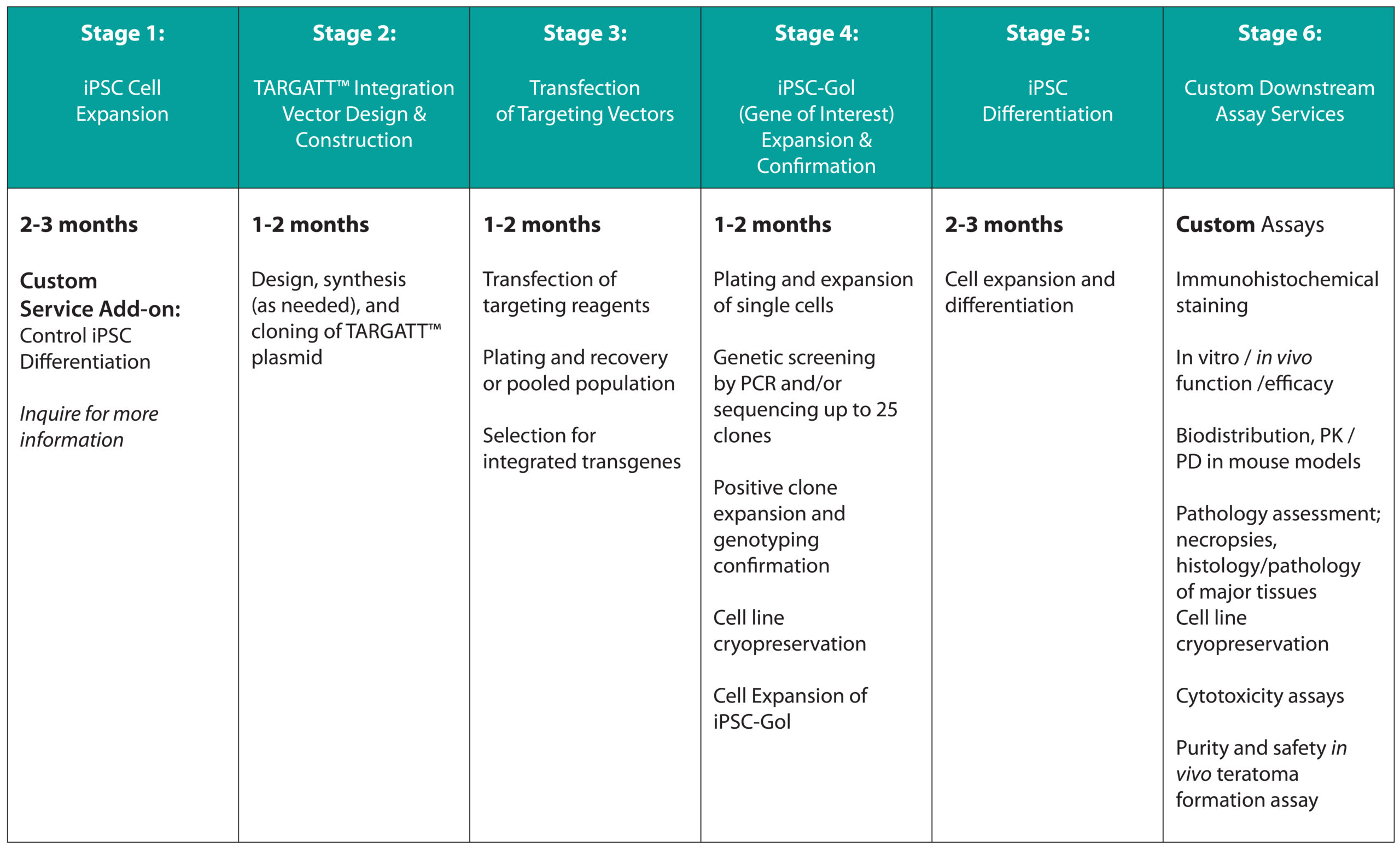
cGMP-Grade TARGATT™ iPSC (Gene Knock-in Ready)
Products and Services
| Catalog ID# | Product Name | Format | Size | Price | QTY |
|---|---|---|---|---|---|
|
|
- |
|
- | ||
|
|
Frozen |
|
- | ||
|
|
Frozen |
|
- | ||
|
|
Service |
|
- |


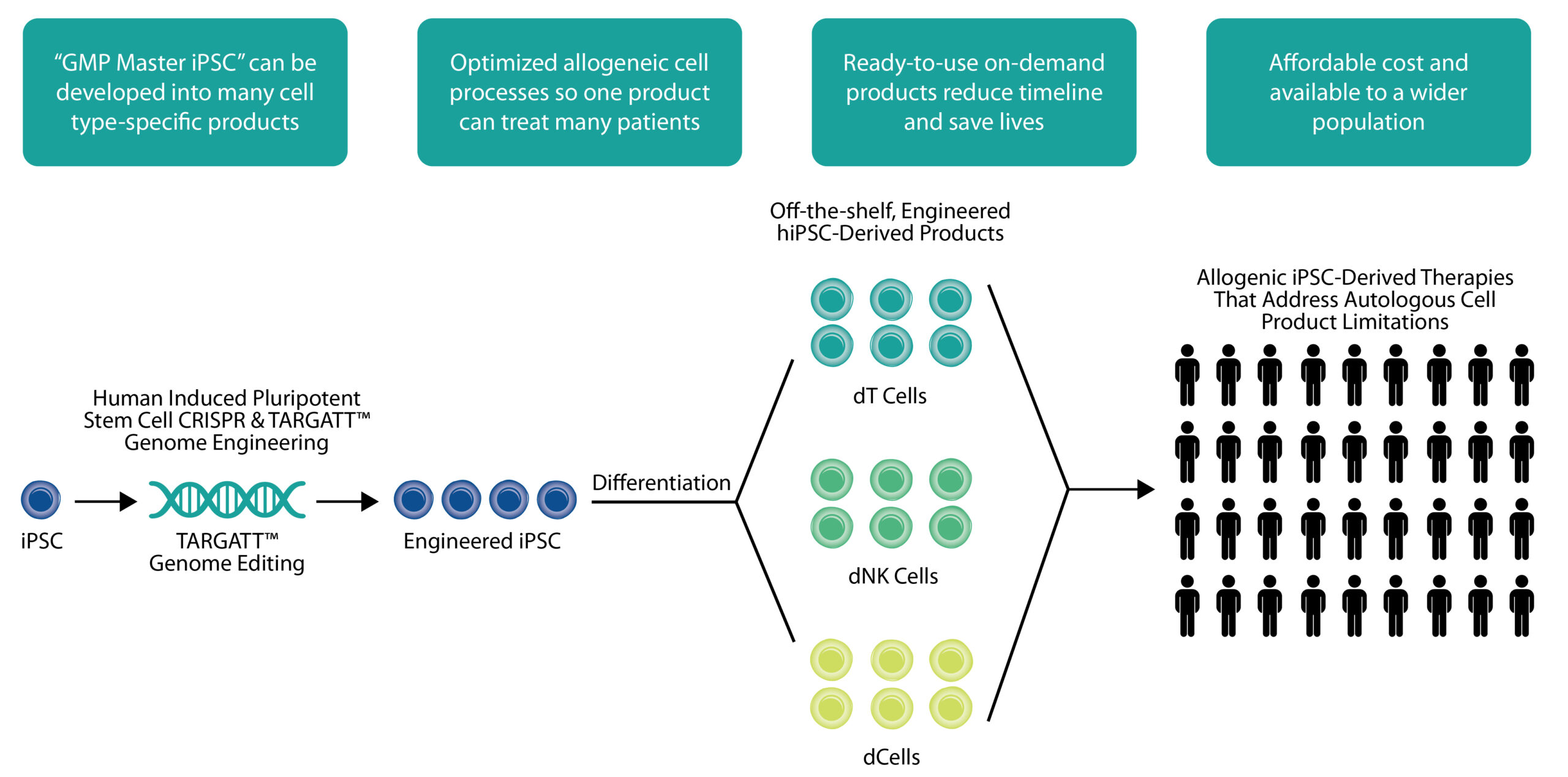 cell therapy approaches include:
cell therapy approaches include: