CRISPR, MAD7 Gene Editing
Gene edited cell and animal models
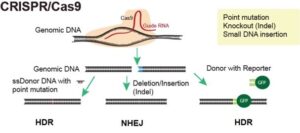 Gene editing is a powerful molecular tool that allows scientists to precisely modify the genetic material of a cell or organism. A diverse array of gene editing platforms has emerged, each offering unique strengths and capabilities. Among the most prominent is the Clustered Regularly Interspaced Palindromic Repeats – CRISPR-associated protein-9 system (CRISPR-Cas9), celebrated for its simplicity, versatility, and efficiency in targeting specific DNA sequences. CRISPR’s programmable RNA guides guide the Cas9 enzyme to precise locations within the genome, where it induces double-strand breaks, enabling targeted genetic modifications.
Gene editing is a powerful molecular tool that allows scientists to precisely modify the genetic material of a cell or organism. A diverse array of gene editing platforms has emerged, each offering unique strengths and capabilities. Among the most prominent is the Clustered Regularly Interspaced Palindromic Repeats – CRISPR-associated protein-9 system (CRISPR-Cas9), celebrated for its simplicity, versatility, and efficiency in targeting specific DNA sequences. CRISPR’s programmable RNA guides guide the Cas9 enzyme to precise locations within the genome, where it induces double-strand breaks, enabling targeted genetic modifications.
CRISPR Gene Editing
CRISPR gene editing is employed in disease modeling and cell therapy through precise manipulation of the genome. In disease modeling, CRISPR enables the introduction of targeted genetic modifications into cellular or animal models, accurately recapitulating disease phenotypes observed in human patients. This process involves designing guide RNAs (gRNAs) that direct the CRISPR-associated Cas9 endonuclease to specific genomic loci harboring disease-associated mutations. Cas9 induces site-specific double-strand breaks, which permits the introduction of desired genetic alterations or the disruption of target genes. Disease models, both cell and animal models, generated using CRISPR allow researchers to study disease mechanisms, screen potential therapeutics, and explore novel treatment strategies.
In cell therapy, CRISPR modifies genes related to immune response, cell proliferation, or target recognition and enables the development of optimized cell therapies tailored to individual patients to boost therapeutic efficacy, safety, and specificity. These engineered cells can be used for cancer immunotherapy, regenerative medicine, and other therapeutic applications, offering promising avenues for personalized medicine and precision healthcare.
Challenges in CRISPR/Cas9 editing, however, include
- Off-Target Effects: One of the primary challenges in CRISPR/Cas9 gene editing is the occurrence of off-target effects, where unintended genetic alterations are introduced at sites other than the target locus. These off-target effects can compromise the accuracy and safety of gene editing applications, raising concerns for clinical translation.
- Delivery Efficiency: Another challenge is delivering gene editing tools, such as CRISPR-Cas9 components, efficiently into target cells or tissues. Cas9 is a large enzyme and it pushes the limit of viral packaging limit such as AAV.
- Limit on payload insertion: CRISPR/Cas9 mediated gene knock-in (KI) is inefficient. This is because the process relies on the host homologous recombination pathway, which by nature happens at an extremely low rate. Furthermore, KI efficiency goes down with increased size of the KI fragment. In general, CRISPR/Cas9 works at reasonable efficiency for fragments less than 2kb in size.
- IP limitation: ASC has CRISPR/Cas9 license for research use. For therapeutic uses, our client will need to obtain such a license for commercialization.
MAD7 Gene Editing
MAD7, or ErCas12a, is a Class 2 Type V Cas nuclease that can be used for gene editing.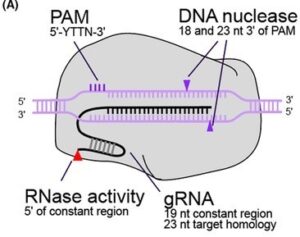 It was developed by Inscripta and is intended to be a royalty-free alternative for academic and industrial research. MAD7 is based on a codon-optimized gene from the Eubacterium rectale and is similar to Cas9 in that it is an RNA-guided nuclease. But different from Cas9, Mad7 naturally employs a single RNA species to guide it to the target DNA sequence and it creates DNA DSB with sticky ends rather than blunt ends. Mad7 displays a preference for a 5′-TTTN-3′ or 5’ –CTTN-3’ PAM site rather than 5′-NGG-3′, which is preferred by Cas9. We have successfully used MAD7 to do gene editing in iPSC lines and HEK293 cells.
It was developed by Inscripta and is intended to be a royalty-free alternative for academic and industrial research. MAD7 is based on a codon-optimized gene from the Eubacterium rectale and is similar to Cas9 in that it is an RNA-guided nuclease. But different from Cas9, Mad7 naturally employs a single RNA species to guide it to the target DNA sequence and it creates DNA DSB with sticky ends rather than blunt ends. Mad7 displays a preference for a 5′-TTTN-3′ or 5’ –CTTN-3’ PAM site rather than 5′-NGG-3′, which is preferred by Cas9. We have successfully used MAD7 to do gene editing in iPSC lines and HEK293 cells.

