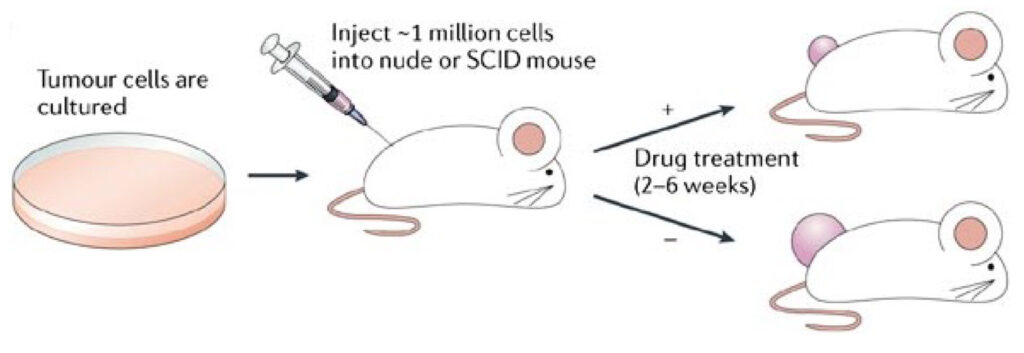
Nonclinical in vivo product efficacy studies
As gene editing technologies evolve, the number of potential engineered cell-based treatments continues to increase. With tools such as CRISPR and TARGATT™™, it is easier than ever before to insert chimeric antigen receptor (CAR) genes into iPSCs that can be further differentiated into natural killer (NK) or T cells or directly into primary T or NK cells. While there is still plenty of cancer immunotherapy research to be done, CAR-NK and CAR-T cell engineering provides a great opportunity for the development of therapeutics that could potentially target and eliminate various forms of cancer. These new cell therapies could be revolutionary, but before entering clinical trials, it is important to ensure these candidates are effective and safe.
Whether you are looking to start your in vitro experiments or in vivo preclinical studies, Applied StemCell can help you design the right efficacy, safety, or toxicity assay for your cancer immunotherapy research. ASC offers a series of customizable CAR-NK and CAR-T cell assay services that capture your requirements and deliver detailed intermediate and final reports with essential data for your preclinical studies. Similar studies are also available for testing biologics products such as antibody drugs.
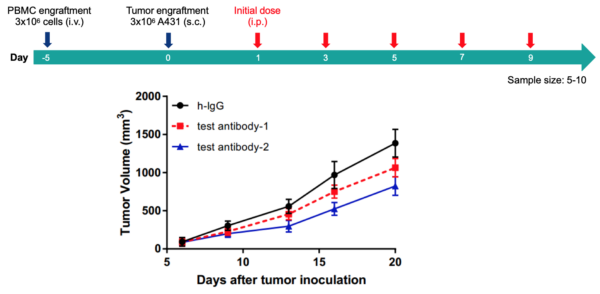
(Figures are adopted from Mol Ther Methods Clin Dev. 2022 Dec 8; 27: 17–31)
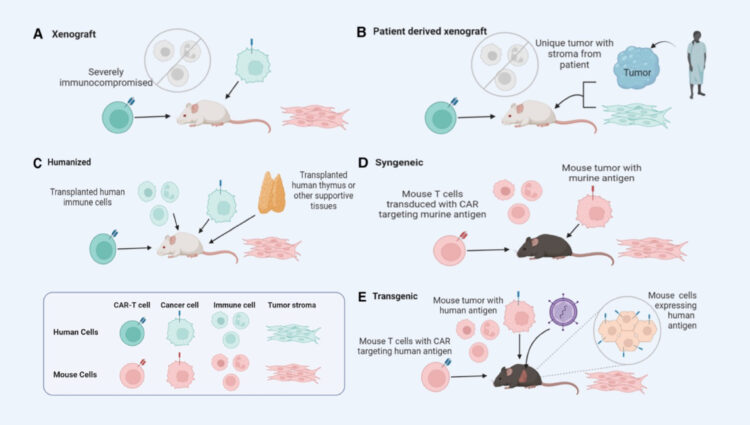
ASC offers various mouse models to serve specific needs of your project. These models include xenograft models, patient cell derive xenograft models, humanized models, syngeneic mouse models, and transgenic models.
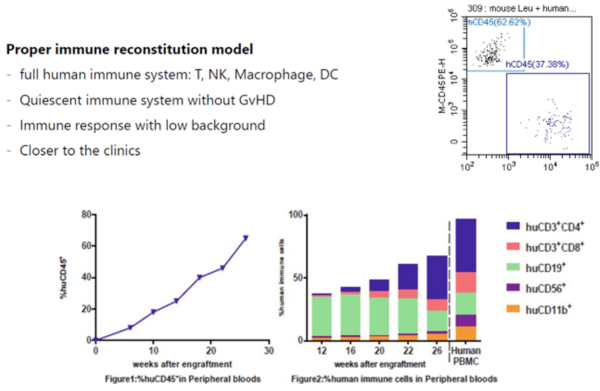
We offer services in generating peripheral blood mononuclear cell (PBMC) or CD34+ hematopoietic stem cells (HSCs) engrafted humanized models by transplanting PBMC or CD34+ HSC into our own immunodeficient NSG animals. These humanized models can be used for in vivo testing of biologics and cell gene products.