Announcing our Drug Master File Submission to the US FDA for our GMP-Grade iPSC Cell Line
April 14, 2025 12:00 PM PST
We are thrilled to announce that we have submitted a Type II Drug Master File (DMF) to the U.S. Food and Drug Administration (FDA) for our ActiCells™ GMP Human Induced Pluripotent Stem Cell (hiPSC) line. This milestone underscores our company’s commitment to empowering scientists to accelerate their cell therapy development by providing high-quality, regulatory-compliant iPSC solutions.
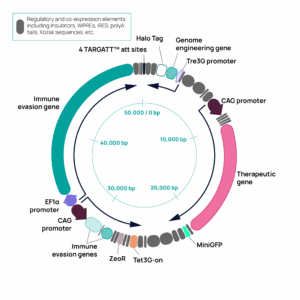
In addition to our ActiCells GMP hiPSC line, we’re introducing a powerful iPSC line pre-engineered with our patented TARGATT™ landing pad at the H11 safe harbor locus. This innovation enables precise, site-specific insertion of as much as 20 kb of DNA in a single reaction, allowing for consistent, reliable expression of one or more genes. By combining GMP compliance with TARGATT™ technology, we offer cell therapy developers an efficient and scalable solution for engineering iPSCs to meet their specific therapeutic needs.
“Securing a DMF for our ActiCells GMP hiPSC line, along with introducing our ActiCells GMP TARGATT™ hiPSC Knock-in Kit, represents a major advancement for the cell therapy industry,” said Ruby Tsai, Ph.D., CEO at Applied StemCell. “By providing a fully characterized, regulatory-ready iPSC platform, we empower researchers to accelerate development while reducing manufacturing and regulatory hurdles.”
Our GMP iPSC solutions support a wide range of regenerative medicine and cell-based therapy applications. With the added advantage of a pre-engineered TARGATT™ landing pad, developers can efficiently generate gene-edited cell lines with high efficiency and reproducibility, expediting the path to clinical and commercial success.
For more information on how our ActiCells GMP hiPSC line can advance your therapeutic development, contact us below.
About Applied StemCell
Founded in 2008 to provide industry and academic researchers with the ability to leverage the power of induced pluripotent stem cell (iPSC) technology, we continue to use our innovative technology to power autologous and allogeneic discovery and development. Our products and services enable a full spectrum of studies from basic research to GMP manufacturing and biobanking—including disease modeling, library construction, drug screening, and nonclinical and pre-IND mouse studies—and our patented technologies ensure a clear IP path to commercialization.
Media Contact
Pia Abola
Director of Marketing
pia.abola@appliedstemcell.com
+1 (408) 457-1312