Easily explore the power of iPSC exosomes for studies on wound healing, aging, nerve regeneration, and more.
Uncover new biological insights with hiEX™ Research iPSC Exosomes— consistent and scalable extracellular vesicles (EVs). These exosomes are isolated from a single, well-characterized human iPSC line, ActiCells™ GMP-Matching RUO TARGATT™ hiPSCs (Cat.#AST-9450), which is grown using proprietary 3D culture conditions optimized for maintaining pluripotency. hiEX™ Research iPSC Exosomes are ideal for probing fundamental biological questions while laying the groundwork for therapeutic innovation.
As central players in cell-cell communication, exosomes can deliver miRNAs, proteins, and other biomolecules that influence cell fate, inflammation, repair, and regeneration1–3.
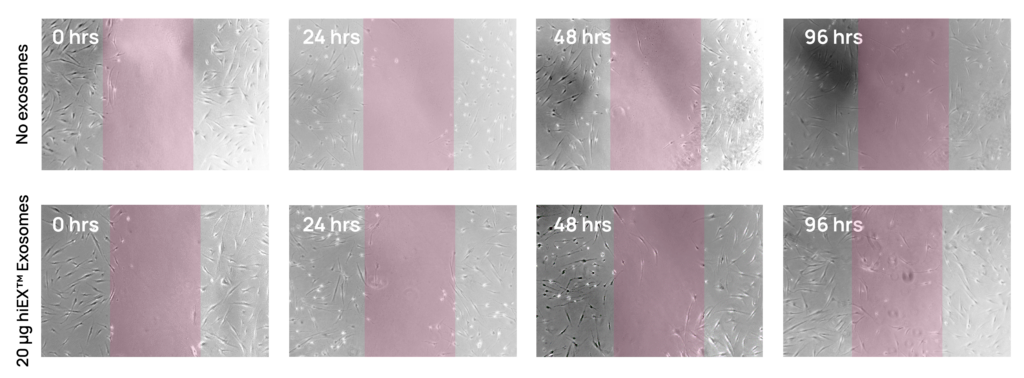
Emerging studies highlight the power of iPSC-derived exosomes to modulate key biological pathways and potentially treat disease1–3. They’ve been shown to enhance neuronal survival and axonal regeneration in spinal cord and nerve crush models4,5, lessen biomarkers of aging and senescence in human dermal fibroblasts6, reduce the vascular remodeling that is the hallmark of pulmonary arterial hypertension (PAH) in a rat model7, and promote wound healing8, including in corneal epithelial defects9. These are just a small sample of existing work, with additional studies being published every day.
hiEX™ exosomes offer a clean and reproducible system for studying cellular physiology and disease treatments free from the donor variability and mixed genetic backgrounds that complicate primary cell-derived preparations like mesenchymal stem cells (MSCs).
Because hiEX™ exosomes are isolated from a cell line that has a TARGATT™ landing pad at the H11 safe harbor site, ActiCells™ GMP-Matching RUO TARGATT™ hiPSCs (Cat.#AST-9450), we can easily express one or more genes-of-interest in the parent cells for incorporation into exosomes. We can also differentiate the parent iPSCs into your desired cell type before isolating exosomes. To learn more about how we can customize your exosomes, visit our iPSC Exosome Services page or contact us.
Whether you’re exploring the fundamental roles of extracellular vesicles or laying the foundation for exosome-based therapeutics, hiEX™ Research iPSC Exosomes deliver the quality, flexibility, and scientific clarity your work demands.
Every vial of hiEX™ Research iPSC Exosomes (Cat.# ASXO-1003) contains >10 x 109 extracellular vesicle (EV) particles isolated from human iPSCs in 1 mL of PBS (custom formulations available—contact us).
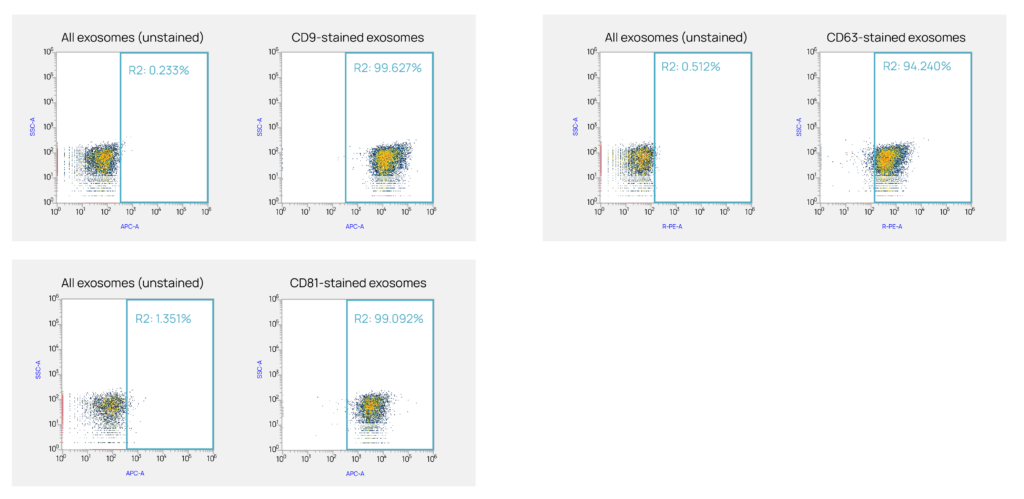
Each lot is rigorously characterized for identity and purity, including tetraspanin surface markers (CD9, CD63, CD81), particle size (typically 30 – 120 nm in diameter), and concentration.
We periodically perform mass spectrometry and RNAseq analysis on hiEX™ Exosomes and can confirm that they contain the expected exosome markers CD9, CD63, CD81, and TSG-101. hiEX™ Exosomes do not contain any Yamanaka reprogramming factors—OCT4, SOX2, c-MYC, and KLF4—indicating that they pose no risk for reprogramming exposed cells.
Representative proteomic and miRNA profiles are available upon request to support your mechanistic studies.
We performed RNA-seq on hiEX™ Research iPSC Exosomes in conditioned medium and found the miRNAs listed below as well as many others.
| miRNA | Activity and/or function | Reference |
|---|---|---|
| miR-302b | Rejuvenation, reversing cellular senescence | Bi Y, Qiao X, Cai Z, et al. Exosomal miR-302b rejuvenates aging mice by reversing the proliferative arrest of senescent cells. Cell Metab. 2025;37(2):527-541.e6. doi:10.1016/j.cmet.2024.11.013 |
| miR-302a | Promotes cognitive improvement and life extension in an animal model of Alzheimer's Disease | Li Y, Sun J, Zheng Y, Xu T, Zhang Y, Wang Y. MiR-302-Induced anti-aging neural stem cells enhance cognitive function and extend lifespan. Published online February 14, 2023:2023.02.13.528232. doi:10.1101/2023.02.13.528232 |
| miR-26a1/2 | Arresting neuronal damage by suppressing apoptosis | Hou Z, Chen J, Yang H, Hu X, Yang F. microRNA-26a shuttled by extracellular vesicles secreted from adipose-derived mesenchymal stem cells reduce neuronal damage through KLF9-mediated regulation of TRAF2/KLF2 axis. Adipocyte. 2021;10(1):378-393. doi:10.1080/21623945.2021.1938829 |
| miR-320a | Preventing ovarian aging | Liu Y, Mu H, Chen Y, et al. Follicular fluid-derived exosomes rejuvenate ovarian aging through miR-320a-3p-mediated FOXQ1 inhibition. Life Med. 2024;3(1):lnae013. doi:10.1093/lifemedi/lnae013 |
| miR-30d | Protect against pathological cardiac hypertrophy by negatively regulating MAP4K4 and GRP78 | Li J, Sha Z, Zhu X, et al. Targeting miR-30d reverses pathological cardiac hypertrophy. eBioMedicine. 2022;81. doi:10.1016/j.ebiom.2022.104108 |
| miR-148a | Promotes cartilage production and inhibits cartilage degradation | Vonk LA, Kragten AHM, Dhert WJA, Saris DBF, Creemers LB. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2014;22(1):145-153. doi:10.1016/j.joca.2013.11.006 |
| miR-21 | Protects against aging-related oxidative damage of CD4+ T cells | Xiong Y, Xiong Y, Zhang H, et al. hPMSCs-Derived Exosomal miRNA-21 Protects Against Aging-Related Oxidative Damage of CD4+ T Cells by Targeting the PTEN/PI3K-Nrf2 Axis. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.780897 |
For a complete list of representative miRNAs found in hiEX™ Research iPSC Exosomes, contact us.
We identified these and many more proteins—including the exosome biomarkers CD9, CD63, CD81, and TSG-101—via mass spectrometry of hiEX™ Research iPSC Exosomes in conditioned medium.
We did not find any Yamanaka reprogramming factors—OCT4, SOX2, c-MYC, and KLF4—indicating that they pose no risk for reprogramming exposed cells.
Proteins in each column are listed from most abundant to least.
For a complete list of representative proteins found in hiEX™ Research iPSC Exosomes, contact us.
Fill out the contact form below and a team member will be in touch within one business day.