Applied StemCell's Brand Refresh: Building the biology-based future
January 30, 2025 12:00 PM PST
Drug discovery is a famously challenging endeavor, with a 96% failure rate, a $1B+ price tag to develop a single drug, and a 10+ year timeline from discovery to commercialization. What if we could invert those numbers? Turn a 96% failure rate into a 96% success rate? Reduce $1B to $100M? Turn 10 years into 10 quarters?
These questions have led to a lot of introspection at Applied StemCell, and they’ve inspired us to double down on our brand identity and recharge our visual identity. The heart of what we do—the TARGATT™ gene knock-in technology that sets us apart and the iPSC expertise that led to the founding of our company—puts us in the ideal position to move the needle on the aforementioned drug discovery challenges.
And so, our new tagline was born:
Biological relevance. Engineered precision. GMP expertise.
Biological relevance
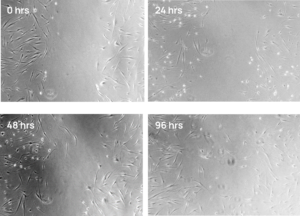
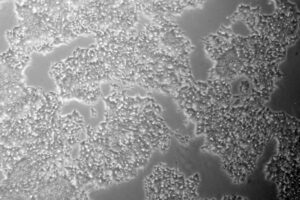
The point where most drugs fail is during the transition into human trials. One of the biggest reasons is that discovery-phase studies don’t simulate human physiology faithfully enough—they lack biological relevance. But with iPSCs, we can build better cell-based models of healthy and diseased human physiology, whether we are talking about cultured cells, organoids, or the nascent 3D printed tissue technology.
With iPSCs, you no longer have to rely on immortalized cell lines that are not reflective of cells in the human body, studies in animals that don’t recapitulate human physiology, or a continuous supply of donated tissue, which can introduce variability from donor to donor.
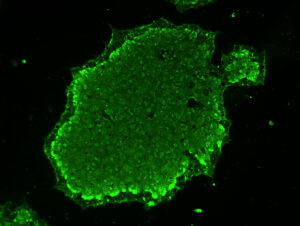
Instead, you can access unlimited amounts of genetically identical cells that are differentiated into your target cell type. Even better, with our gene editing expertise and exclusive technologies, we can engineer iPSCs to better replicate the disease state you are studying.
Our TARGATT™ technology also inverts the drug discovery challenge by streamlining antibody- and protein-based therapeutic screening and studies. The large cargo insertion capability and high-efficiency of TARGATT™ knock-in means that you can screen antibodies in more biologically relevant cells—the same cells you will use to produce the antibodies. You save time and, therefore, money while reducing the risk of advancing a non-developable candidate.
Engineered precision
We add “precision” to our cell engineering thanks to the site specificity of our TARGATT™ knock-in technology and the negligible off-target events delivered by our AccuBase base editing technology. CRISPR is a revolutionary technology, but it’s not the right solution for every problem. Especially for developing cell-based therapies, you need to ensure safety by eliminating the risk of mutagenesis from off-target events.
With our unique and innovative technologies, we ensure that every cell line is precision-engineered.
GMP expertise
We add GMP expertise to our tagline to emphasize the importance of successfully navigating quality and regulatory challenges to maximize the impact of your innovation. For your projects, we leverage the same GMP facilities and regulatory expertise used to manufacture our GMP iPS cell lines.
Reaching for 96% success—inverting the drug failure paradigm
Ultimately, increasing the drug candidate success rate and speeding up development times is about maximizing the impact the medicines we discover and develop will have on human health. By reducing R&D costs we can lower the price an individual patient will pay, enabling wider access to treatments and building a healthier world.
At Applied StemCell, we’re leading the charge through biological relevance, engineered precision, and GMP expertise.