A quick overview of this industry-changing technology as well as the newer Cas9 variants that expand its use
As a company that was founded before CRISPR/Cas9 became a commercial genome editing tool—we were one of the first to license its use in cells and rodents in 2014—we can attest to the game-changing power of the technology. Suddenly, customizing genomes and engineering cell lines became a straightforward and reliable exercise that most molecular biology labs could do.
Now that we’re over a decade past its initial discovery, CRISPR/Cas9 continues to be an incredibly valuable tool, with new Cas9 variants and novel CRISPR-like systems expanding what the technology can accomplish.
Here, we review the basics of the CRISPR/Cas9 system and the Cas9 variants—CRISPRi and CRISPRa—that we use at Applied StemCell to enable promoter studies and cancer research.
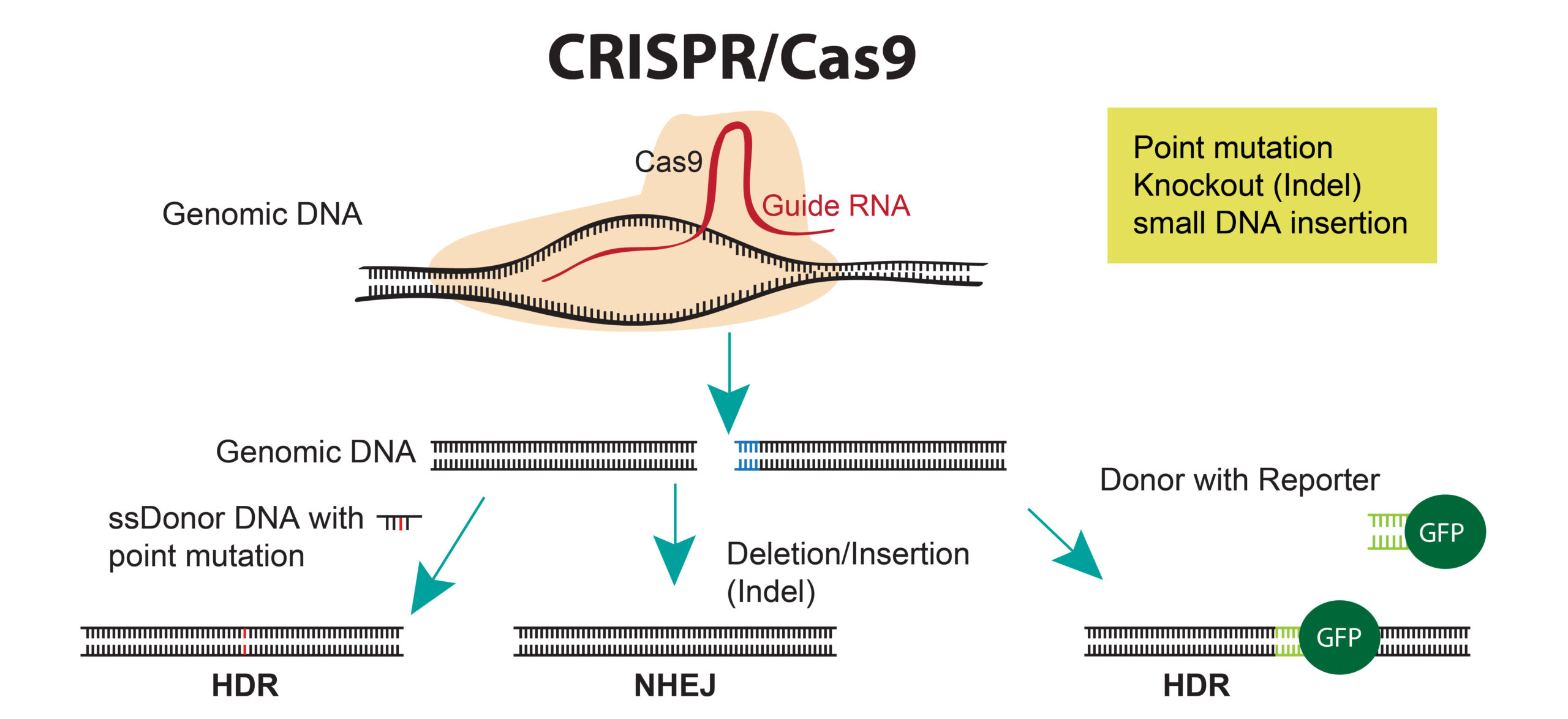
Cas9 is a site-specific nuclease that uses a small guide RNA to target the Cas9 nuclease to a specific sequence in the genome. In bacteria, Cas9 uses two RNAs, a CRISPR RNA (crRNA) that targets the nucelase to a specific sequence and a transactivating CRISPR RNA (tracrRNA) that binds to the crRNA and activates the CAS9 nuclease. For convenience in the lab, the crRNA and tracrRNAs are fused by a linker into a single guide RNA (gRNA).
Wild-type Cas9 creates double stranded breaks in the genome that can be repaired using non-homologous end joining (NHEJ)—creating an indel (insertion/deletion)—or via homologous recombination with a donor plasmid (homology directed repair (HDR)) to create knock-ins or point mutations.
A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity
Martin Jinek, Krzysztof Chylinski, Ines Fonfara, Michael Hauer, Jennifer A. Doudna, and Emmanuelle Charpentier
Science. 28 Jun 2012. 337(6096):816-821
DOI: 10.1126/science.1225829
Multiplex Genome Engineering Using CRISPR/Cas Systems
Le Cong, F. Ann Ran, David Cox, Shuailiang Lin, Robert Barretto, Naomi Habib, Patrick D. Hsu, Xuebing Wu, Wenyan Jiang, Luciano A. Marraffini, and Feng Zhang
Science. 3 Jan 2013. 339(6121):819-823
DOI: 10.1126/science.1231143
By modifying the Cas9 protein, scientists have turned an already powerful technology into one that works better and does more. At Applied StemCell, we leverage a number of Cas9 variants to ensure that we achieve your goals. Furthermore, we will always recommend the most appropriate technology for your application based on our extensive cell line engineering experience.
Off-target events and low edit efficiency are two of the biggest challenges when working with CRISPR/Cas9.
Fusion proteins can help.
Enhanced Knock-in Efficiency
Reduced off-target events
By stimulating HDR, both fusion proteins reduce NHEJ, thus reducing the occurrence of random repair and off-target edits.
The ability to direct an enzyme to a specific genomic location by simply creating a guide RNA is what makes the CRISPR/Cas9 system so powerful.
Knocking out Cas9’s nuclease activity and fusing the protein to activators and repressors turns a gene editing enzyme into a site-directed transcription control agent.
Repressing transcription
CRISPRi fuses a catalytically inactive Cas9 to transcriptional repressors, such as KRAB (Krüppel-associated box) to provide site-specific silencing of gene expression.3
Activating transcription
CRISPRa fuses a catalytically inactive Cas9 to transcriptional activators, such as the VPR system (VP64-p65-Rta), to assemble gene transcription machinery at a specific site.4
Using Cas9 variants expands what the Applied StemCell team can help you accomplish. Here are just a few examples:
Study disease in relevant cell types with full confidence that the phenotype you observe is not due to an off-target mutation, whether you are knocking in a functional gene, knocking out a gene, or exploring mutations in non-coding regions.
Explore gene function and map out gene interactions, genetic pathways, and complex gene networks with clarity and confidence.
Fill out the contact form below and a team member will be in touch within one business day.