Complete GMP iPSC & MSC Platform
Gene and cell therapies are paradigm changing, new therapeutic genomic medicine. This makes the CMC and regulatory landscape quite complex to navigate successfully compared to small or large molecule-based therapies. Especially in cell manufacturing, you may encounter various challenges, but you don’t have to face them alone. Our GMP experts can guide you through every step of the process from technology transfer, engineering run, analytical method qualification, scale-up, and GMP production. We can help you develop your project outline and ensure you receive detailed updates as we collaborate on your project until it is completed.
At our cGMP-compliant facility, we offer custom cell therapy manufacturing service options, including:
Our team can help you overcome CMC challenges and has the experience to advise on operational, logistic, quality and regulatory considerations for successful IND / CTA submissions and clinical supply. Our cGMP facilities are compliant for:
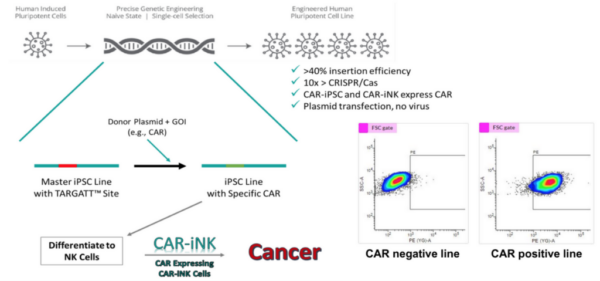
Our one-stop cGMP-compliant, iPSC Cell Product platform spans the whole range of iPSC related activities from iPSC generation, gene editing, differentiation and further to cryopreservation and cell banking.

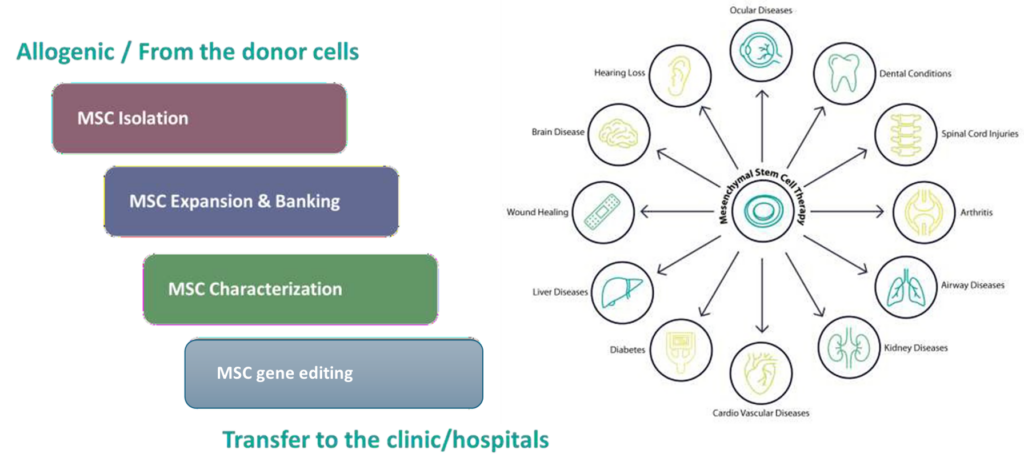
Mesenchymal stem cells are a popular choice for cell therapy starting material due to their potential to differentiate into multiple lineages. We offer end-to-end stem cell solutions, including cell isolation, expansion and banking, characterization, and differentiation.
We also offer primary cell isolation, cell banking and cell product manufacturing, featuring primary T cells, NK cells, monocytes, hematopoietic stem cells, etc.