Applied StemCell Demonstrates Efficient Knock-In of a 50 kb DNA Construct into Human iPSCs Using TARGATT™ Technology
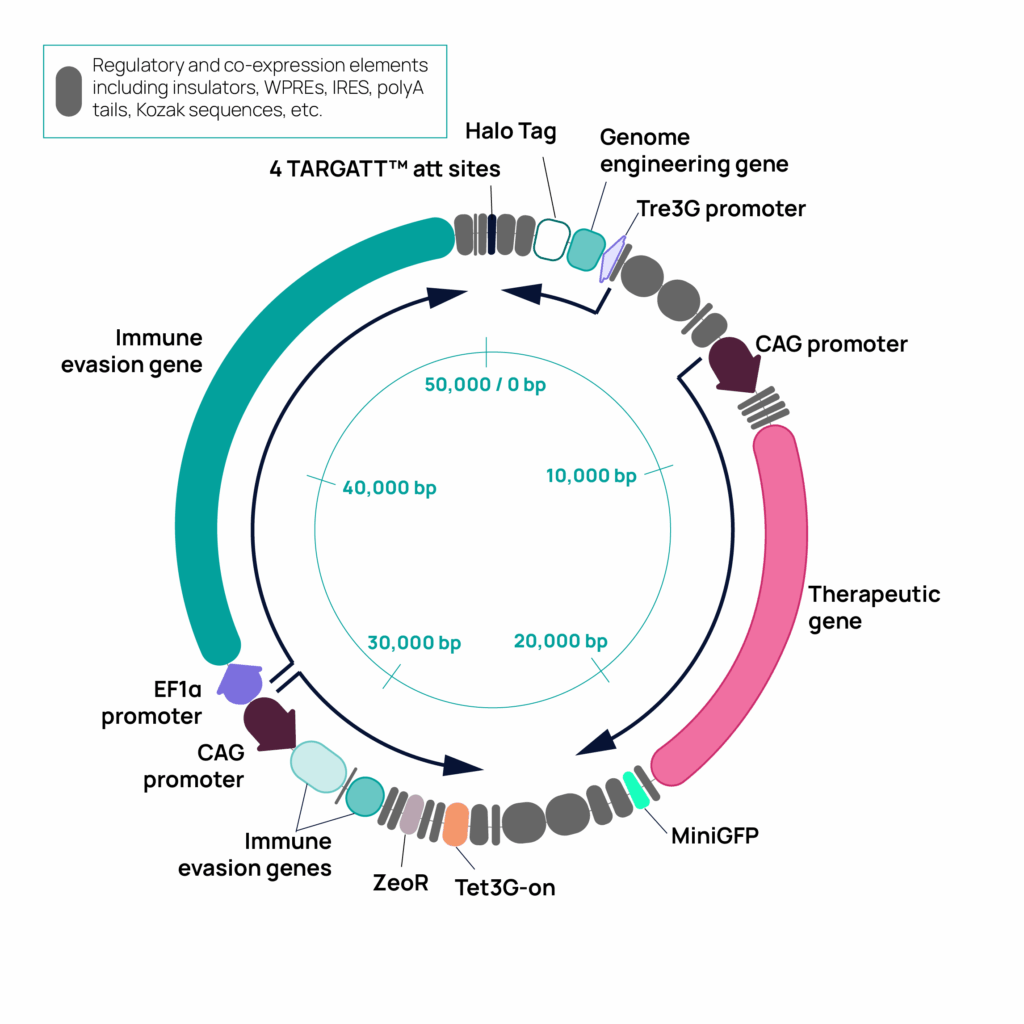
Applied StemCell demonstrates efficient knock-in of a 50 kb DNA construct into human iPSCs using TARGATT™ technology November 11, 2025 8:00 AM PST Milpitas, CA — Applied StemCell, Inc. (ASC), today announced the successful knock-in of a 50 kilobase (kb) multifunctional DNA insert into human induced pluripotent stem cells (iPSCs) using its proprietary TARGATT™ technology. […]
Welcome to the team, Kaytrina!

Celebrating our growing Business Development team September 17, 2025 11:00 AM PST We’re thrilled to welcome Kaytrina Anchelia as a Director of Business Development. With nearly a decade of experience driving significant business growth at OmniaBio, iVexSol, and Akron Bio, she brings deep commercial expertise in the cell and gene therapy space. Kaytrina will leverage […]
Applied StemCell Appoints Dolores Baksh, Ph.D., as Chief Executive Officer

Applied StemCell Appoints Dolores Baksh, Ph.D., as Chief Executive Officer August 21, 2025 10:00 AM PST Applied StemCell, a genome engineering product and services company, announced today the appointment of Dolores Baksh, Ph.D., as Chief Executive Officer. Company founder and former CEO Ruby Tsai, Ph.D., will continue to guide scientific strategy, innovation, and strategic collaborations […]
Our two new hypoimmunogenic hiPSC products are here to accelerate your allogeneic therapies
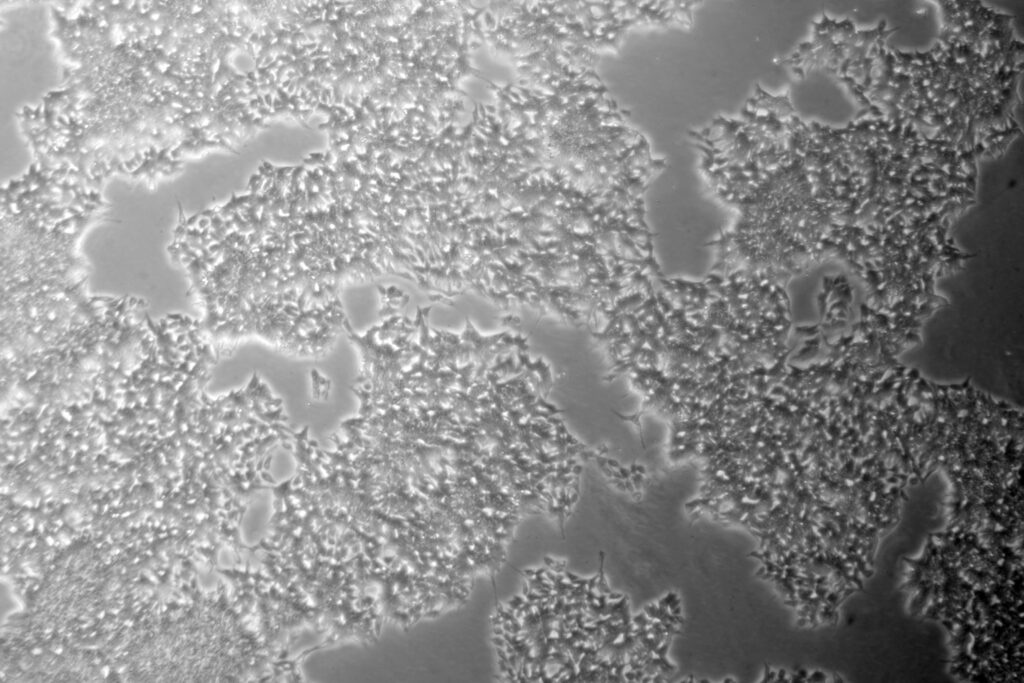
Our two new hypoimmunogenic hiPSC products are here to accelerate your allogeneic therapies May 20, 2025 8:30 AM PST We are thrilled to announce the launch of our first hypoimmunogenic hiPSC products, which are designed to empower researchers developing next-generation allogeneic therapies. The ActiCells™ RUO Hypo hiPSCs (Cat.# ASE-9550) and the ActiCells™ RUO TARGATT™ Hypo […]
Announcing our Drug Master File Submission to the US FDA for our GMP-Grade iPSC Cell Line
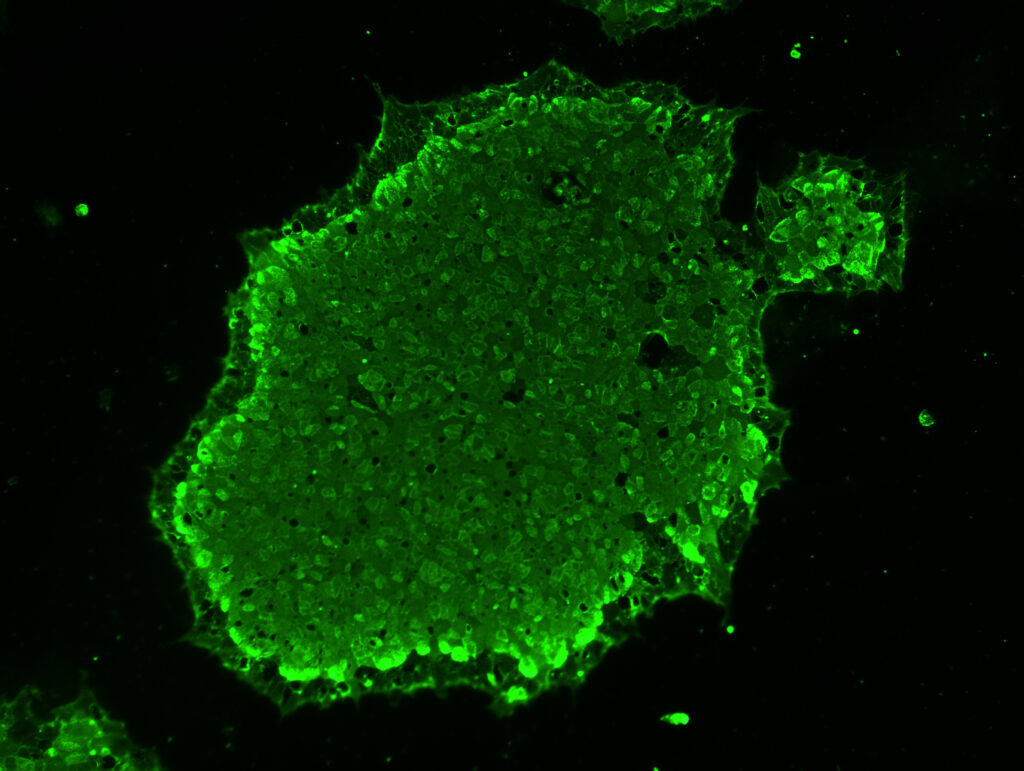
Announcing our Drug Master File Submission to the US FDA for our GMP-Grade iPSC Cell Line April 14, 2025 12:00 PM PST We are thrilled to announce that we have submitted a Type II Drug Master File (DMF) to the U.S. Food and Drug Administration (FDA) for our ActiCells™ GMP Human Induced Pluripotent Stem Cell […]
Applied StemCell Brand Refresh: Building the biology-based future

Applied StemCell’s Brand Refresh: Building the biology-based future January 30, 2025 12:00 PM PST Drug discovery is a famously challenging endeavor, with a 96% failure rate, a $1B+ price tag to develop a single drug, and a 10+ year timeline from discovery to commercialization. What if we could invert those numbers? Turn a 96% failure […]
Applied StemCell welcomes two seasoned leaders to our team

January 14, 2025 9:00 AM PST Milpitas, California, January 2025 – Applied StemCell, Inc., a proven CRO/CDMO and gene editing technology developer, is pleased to welcome Todd Garland to our Board of Directors and Mike Yurkovich as Vice President of Sales. Todd Garland brings over 30 years of expertise in product management, sales, and commercial […]
New Breakthrough in Cell Transplantation Therapy: Applied StemCell’s Patent for HLA-Engineered Universal Donor Cells Approved
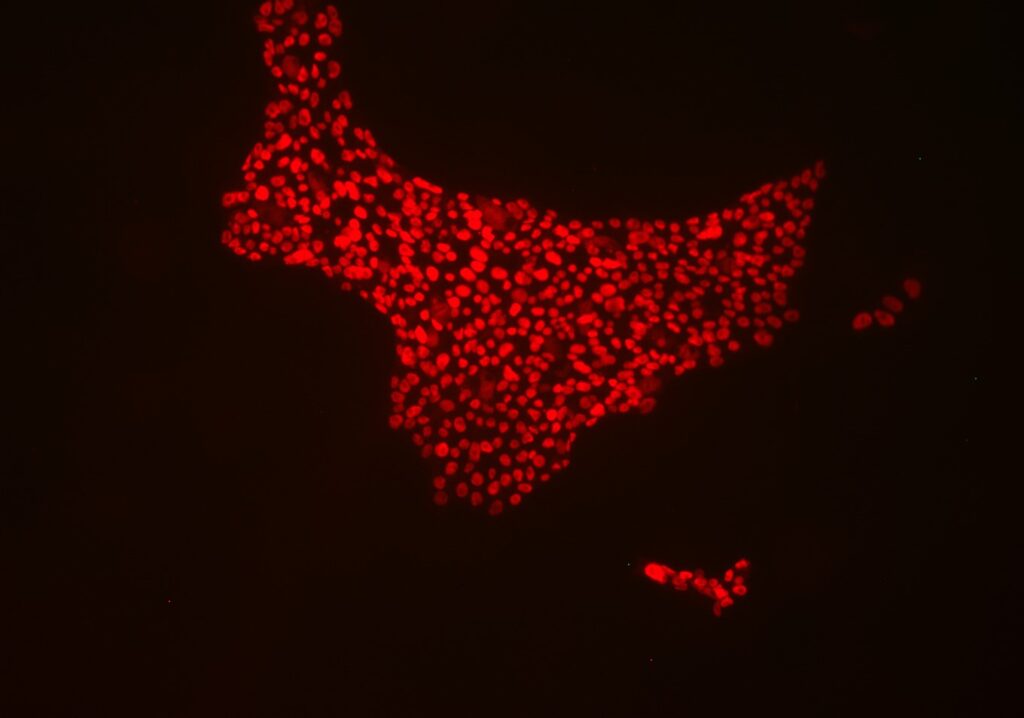
October 01, 2024 10:00 AM PST MILPITAS, Calif. – Applied StemCell, Inc. is proud to announce the successful approval of its latest U.S. patent which covers HLA-F engineered universal donor cells, marking a significant advancement in cell transplantation therapy and the first step towards an allogeneic cell therapy development platform. This innovation not only […]