Easily move from optimization to Advance toward IND-enabling studies: Rely on GMP-grade cells backed by a U.S. FDA Drug Master File (DMF) for easier regulatory alignment.
The TARGATT™ GMP hiPSC Knock-in Kit (AST-9480) empowers you to confidently advance from proof-of-concept to IND-enabling studies with a U.S.-manufactured, clinical-grade cell line. With a parental line that’s covered by a U.S. FDA drug master file (DMF), AST-9480 offers a robust foundation for building your engineered iPSC-derived cell product.
Built with a TARGATT™ landing pad pre-integrated at the H11 safe harbor locus, this hiPSC line supports site-specific, single-copy insertion of payloads up to 20 kb—and more with nested constructs. Whether you’re developing large therapeutic payloads, complex synthetic circuits, or immune-modulating elements, the TARGATT™ system delivers reliable, stable expression without the risks of random integration.
TARGATT™ GMP hiPSCs are isogenic with TARGATT™ RUO hiPSCs, enabling seamless transfer of protocols and data from early-stage development to GMP-grade production. For teams looking to accelerate development with customized edits or directed differentiation, we also offer end-to-end iPSC gene editing services in a GMP environment.
Derived from CD34+ cord blood cells, these hiPSCs start with a lower mutational burden and broader developmental potential than adult somatic sources, making them a superior starting point for applications that require genomic stability and flexible lineage differentiation. In addition, the parental hiPSC line used for AST-9480 has been successfully differentiated into cardiomyocytes, natural killer (NK) cells, T cells, monocytes/macrophages, and endothelial cells—demonstrating its versatility across a range of lineages.
With clear freedom-to-operate and straightforward licensing terms, the TARGATT™ GMP hiPSC Knock-in Kit streamlines your path from development to commercialization.
Each TARGATT™ GMP hiPSC Knock-in Kit contains sufficient cells and plasmids for 3 transfections and comes with:
As with all TARGATT™ technology-enabled products, this kit requires a license for commercial use and a materials transfer agreement (MTA) for academic use.
TARGATT™ large knock-in technology leverages a site-specific serine integrase to unidirectionally insert plasmid DNA sequences into the genome at the TARGATT™ landing pad—learn more by visiting the TARGATT™ Technology page.
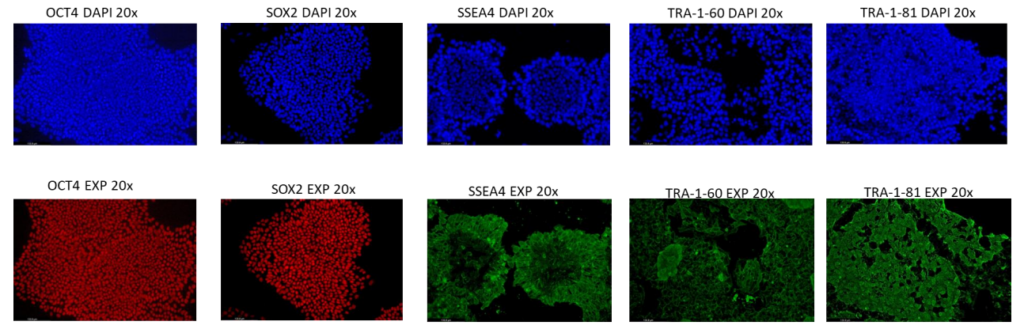
Supporting data shown is for the isogenic TARGATT™ RUO hiPSCs. Data for the GMP line is available upon request.
TARGATT™ RUO hiPSCs are pluripotent
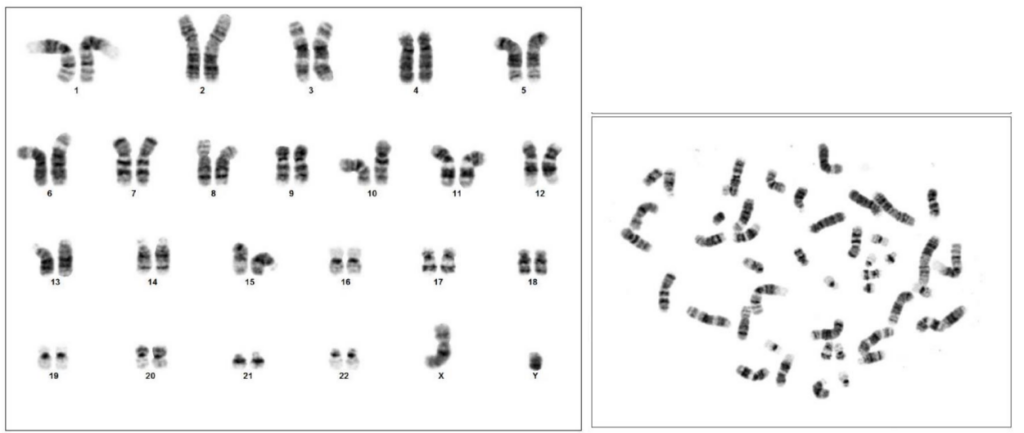
TARGATT™ RUO hiPSCs possess a normal karyotype
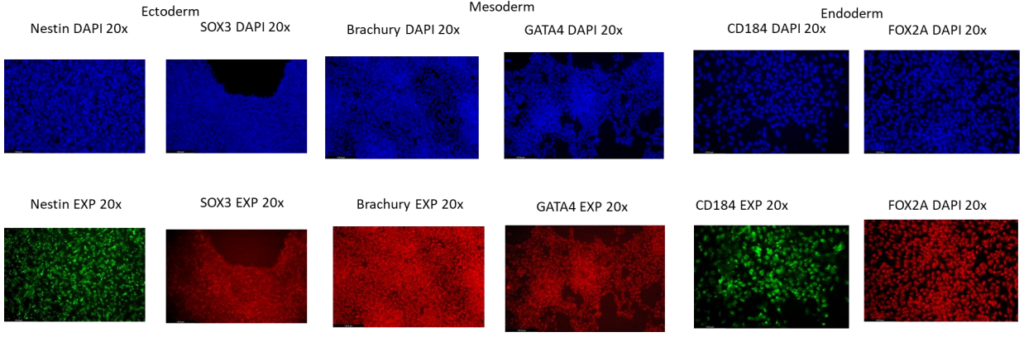
TARGATT™ RUO hiPSCs differentiate to the three germ layers
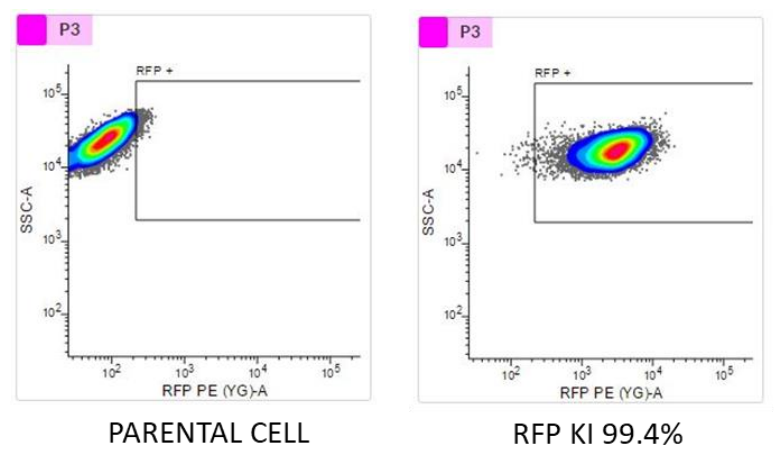
TARGATT™ RUO hiPSCs stably express an inserted transgene, red fluorescent protein (RFP)
Didn’t find all the info you were looking for?
Still have questions?
No problem.
Yes! The TARGATT™ GMP hiPSC Knock-in Kit is manufactured under cGMP and can be used for clinical applications.
The TARGATT™ GMP iPSC Master Cell Line is engineered from our ActiCells™ GMP hiPSC Line (Cat.# ASE-9280), a well-characterized, karyotype-normal hiPSC line reprogrammed from CD34+ cord blood cells from a fully consented male donor.
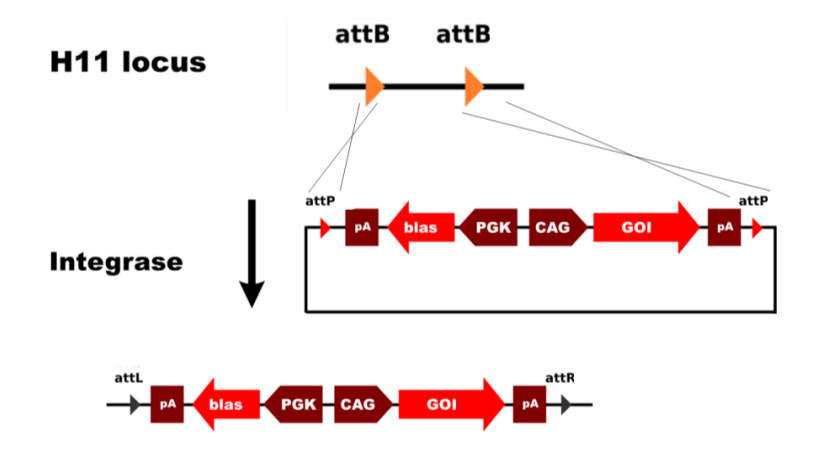
TARGATT™ technology enables highly efficient, single-copy, site-specific integration at the H11 safe harbor locus. Integration is stable and results in long-term expression—advantages that other random or multi-copy insertion methods cannot consistently provide.
TARGATT™ technology provides high knock-in efficiency with stable and site-specific gene integration. After drug selection, efficiency can approach 90%.
The TARGATT™ system uses a patented serine integrase that specifically recognizes TARGATT™ attB sites in the cloning plasmid (also referred to as the donor plasmid) and attP sites in the TARGATT landing pad. Because unbound double strand breaks are not created, random integration using the host double strand break repair machinery does not occur.
TARGATT™ can efficiently insert payloads of 20 kb in a single reaction and larger payloads with nested TARGATT™ landing pads, making the technology suitable for a wide range of genetic engineering applications.
Yes, the TARGATT™ cloning plasmid allows you to clone and amplify your desired gene of interest for site-specific insertion.
Fill out the contact form below and a team member will be in touch within one business day.